ru2+ electron configuration|What is the ground state electron configuration for Ru2 : Tagatay Well, if you are wondering about the electron configuration of Ruthenium then you are at the correct place. The electron configuration of Ruthenium is [Kr] 4d7 5s1 in its short standard form. . Our EPL predictions take an inside look at the teams likely to make the Champions League spot for the coming season. As you might expect, City should lead every list of qualifying teams. Guardiola’s men shouldn't have a problem getting the spot. Then we've got Arsenal who would want to make a statement of intent by trying to win the league .
PH0 · What would the Electronic Configuration of Ru²⁺ be?
PH1 · What is the ground state electron configuration for Ru2
PH2 · Ruthenium Electron Configuration:Everything You Need to Know
PH3 · Ruthenium Electron Configuration (Ru) with Orbital
PH4 · Ruthenium (Ru)
PH5 · Ruthenium
PH6 · Electron configuration of Ruthenium
PH7 · Electron Configuration for Ruthenium
PH8 · Electron Configuration For Ru
PH9 · Complete Electron Configuration of Ruthenium (Ru, Ru3+)
Select and save your numbers and game options on the Louisiana Lottery Official Mobile App and show the resulting code at checkout for your ticket to print. Ask the Retailer. If you want a Quick Pick, tell the clerk. You can play up to 10 Quick Picks on one ticket. These will be $1 Straight plays.
ru2+ electron configuration*******What is the electronic configuration of RuX2+ R u X 2 +? If I were to remove from the highest energy level it would be [Kr] 5sX1 4dX5 [ K r] 5 s X 1 4 d X 5 leaving 6 6 .
Electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 4d xy 1 4d yz 2 4d xz 1 4d 1 x2-y2 4d z2 1 5s 2.As we excite the Ru atom, the electron .Watch on. Key Takeaways: Ruthenium is a transition metal with atomic number 44. The electron configuration of ruthenium is 1s2 2s2p6 3s2p6d10 4s2p6d7 5s1. Ruthenium .
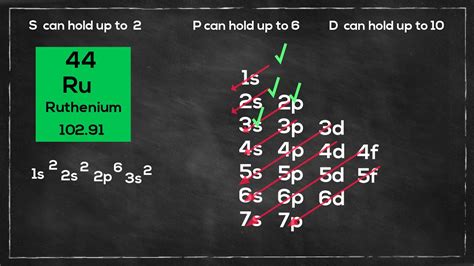
The simplified electron configuration of ruthenium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d7 5s1. Adding all these indices, the total is 44, which is the total number of electrons it has, which is equal to its atomic number. . Well, if you are wondering about the electron configuration of Ruthenium then you are at the correct place. The electron configuration of Ruthenium is [Kr] 4d7 5s1 in its short standard form. .
The electron configuration of ruthenium is 1s2 2s2p6 3s2p6d10 4s2p6d7 5s1. This arrangement of electrons illustrates how they are distributed within the atomic orbitals. In .
Ruthenium atoms have 44 electrons and the shell structure is 2.8.18.15.1. The ground state electron configuration of ground state gaseous neutral ruthenium is [ Kr ]. 4d75s1 and the term symbol is 5F5. .Electron affinity: 100.96 kJ/mol: Oxidation states: −4, −2, 0, +1, +2, +3, +4, +5, +6, +7, +8 (a mildly acidic oxide) Ionization energies: 710.2 kJ/mol; 1620 kJ/mol; 2747 kJ/molMarch 23, 2023. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also .
Sarah Faizi (University of California Davis) 2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is . The electron configuration of the Ruthenium is a highly useful property of the chemical element. Feel free to share the article with others who want to explore this chemical element Ruthenium . Filed . Electron Configuration: 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 7 5s 1; . Valence Electrons: 4d 7 5s 1 Electron Dot Model. Chemical Properties of Ruthenium. Electrochemical Equivalent: 1.257g/amp-hr; Electron Work Function: 4.71eV; Electronegativity: 2.2 (Pauling); 1.42 (Allrod Rochow) Heat of Fusion: 24kJ/mol; .
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer. Question: Predict the ground-state electron configuration of each ion. Use the abbreviated noble gas notation. Ru2+: [Kr]4d6 [Xej4f14 503. Show transcribed image text. There are 3 steps to solve this one.The electronic configuration of an atom of an element is defined as the distribution of its electrons in the respective orbitals or subshell. In doing so Aufbau principle, Hund's rule, Pauli Exclusion rules, etc are followed. . Predict the ground-state electron configuration of Ru2+. Write your answer in abbreviated form, that is, beginning .The elements that form bonds by donating electrons are called cation. The rhodium atom donates an electron in the 5s orbital and two electrons in the 4d orbital to convert a rhodium ion (Rh 3+ ). Rh – 3e – → Rh 3+. The electron configuration of rhodium ion (Rh 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 6.
ru2+ electron configurationThe Ground State of Multi-electron Atoms/Ions. Most of the atoms and ions you will be dealing with are multi-electron species. In atoms/ions with two or more electrons, the ground state electron configuration must (1) minimize the total energy of the electrons, (2) obey the Pauli exclusion principle (3) obey Hunds rule of maximum multiplicity, and (4) .What is the ground state electron configuration for Ru2 The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state . This handy chart compiles the electron configurations of the elements up through number 104.The electron configuration of ruthenium is [Kr].4d 7 5s 1. Ruthenium belongs to the transition metals and is part of group 8 in the periodic table. It has an atomic weight of 101.072 u and a silvery white metallic appearance. The shell structure of ruthenium is 2.8.18.15.1. Ruthenium is a solid at room temperature.Transition Metals and Coordination Compounds 3h 14m. Give the ground state electron configuration and number of unpaired electrons in a Ru2+ ion. (LO 6.1, 6.2) (a) 3Kr45s2 4d4 0 unpaired electrons (b) 3Kr45s2 4d6 0 unpaired electrons (c) 3Kr44d6 4 unpaired electrons (d) 3Kr45s24d4 4 unpaired electrons.
Solution. 1. Locate the atom on the periodic table. 2. Locate the noble gas element in the period above the element of interest. 3. Continue the electron configuration from the noble gas until you reach the element of interest. 4. Put the noble gas in brackets and write the remainder of the electron configuration.
Électrons et configuration électronique. Le nombre d’électrons dans un atome électriquement neutre est le même que le nombre de protons dans le noyau. Par conséquent, le nombre d’électrons dans l’atome neutre de ruthénium est de 44. Chaque électron est influencé par les champs électriques produits par la charge nucléaire .
The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .
ru2+ electron configuration What is the ground state electron configuration for Ru2The electron configuration of ruthenium is 1s2 2s2p6 3s2p6d10 4s2p6d7 5s1. Ruthenium has a hexagonal crystal structure with an atomic radius of 1.89Å. The valence electrons of ruthenium are in the 4d7 and 5s1 orbitals. Determining the electron configuration of ruthenium follows the periodic table and electron filling rules. Ruthenium Atomic .Answer- Ru stands for Ruthenium with atomic number 44. It is a metal and thus, has ability to lose ele .. Predict the ground-state electron configuration of each ion. Use the abbreviated noble gas notation. Ru2+. Electron configurations are are shorthand descriptions of the arrangements of electrons in atoms. An example electron configuration with its general structure is shown in Figure 2.7.1 2.7. 1. In electron configurations, we use numbers to indicate which shell an electron is in. Figure 2.7.1 2.7. 1: General structure of electron .
Welcome to Jonny Jackpot - an online casino for everyone! Jonny warmly welcomes you to begin an incredible online casino journey. We’re a brand-new casino, launched in 2018 and powered by some of the most .
ru2+ electron configuration|What is the ground state electron configuration for Ru2